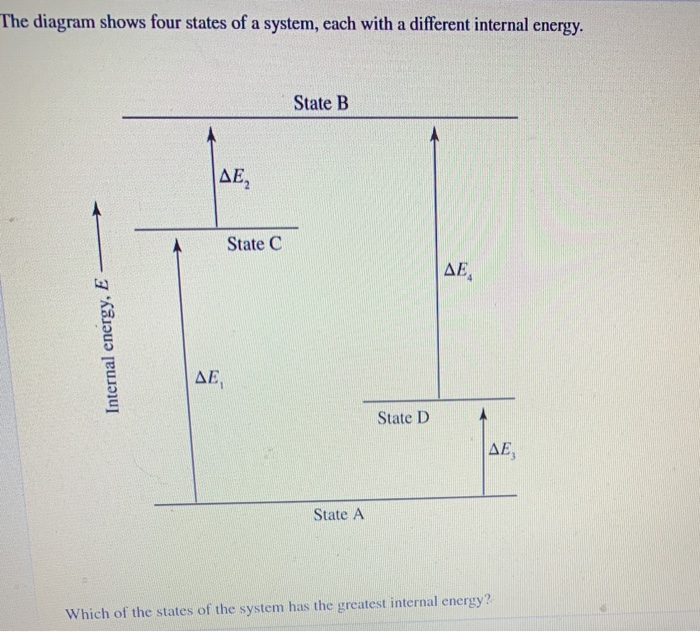
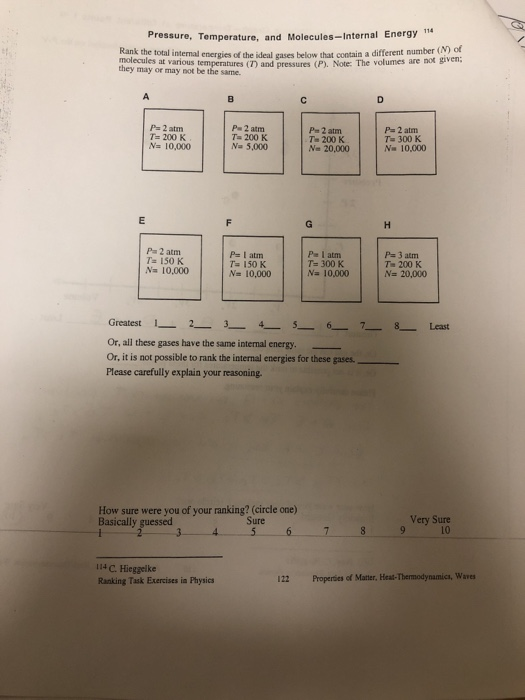
Which Of The States Of The System Has The Greatest Internal Energy?
Which of the states of the system has the greatest internal energy?. ΔU isolated system 0. You probably want the answer to What forms can energy have The answer is this. A system such as your food contains chemical energ.
Kinetic energy Gravitational Energy Chemical Energy Heat Thermal Energy Nuclear Energy Electrical Energy Magnetic energy and many others. A is the sum of the kinetic energy of all of its components. Work is thus done on the system w0.
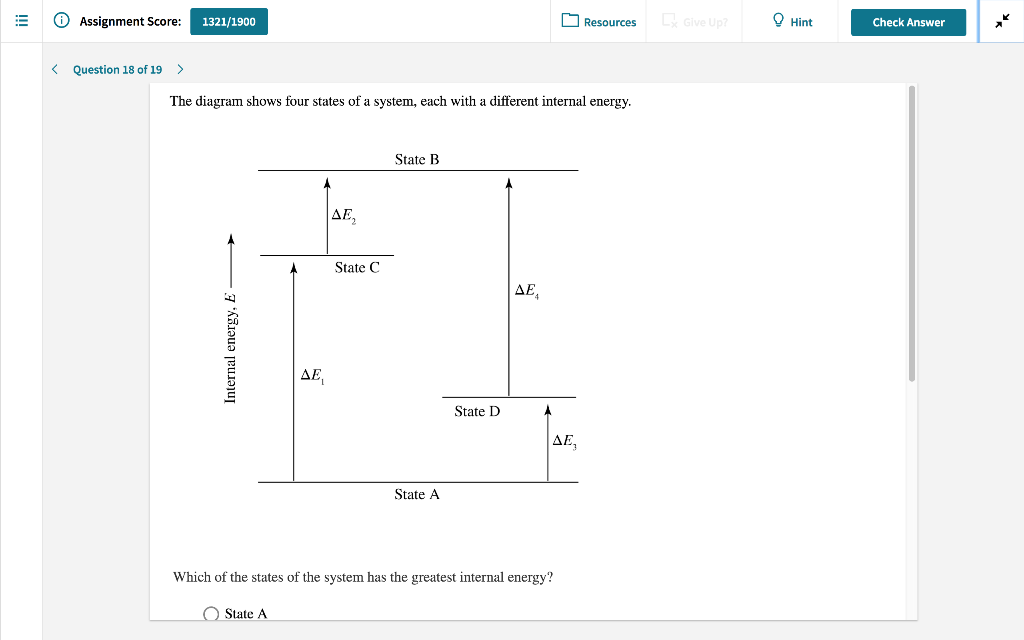
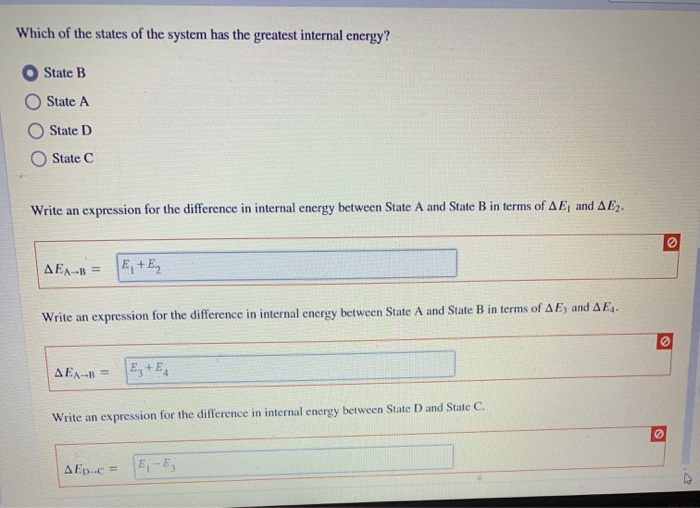
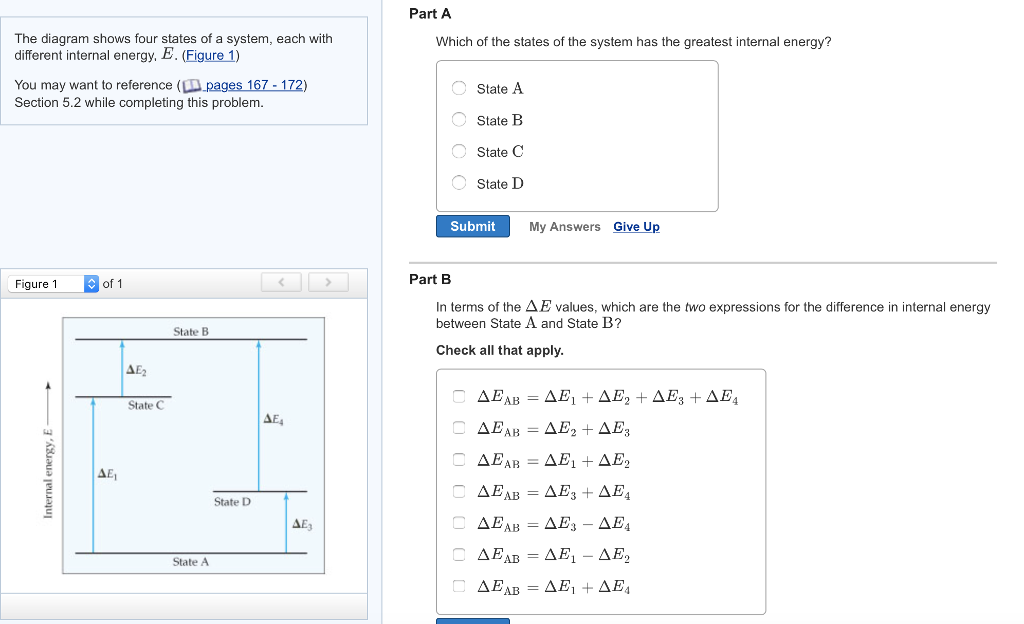
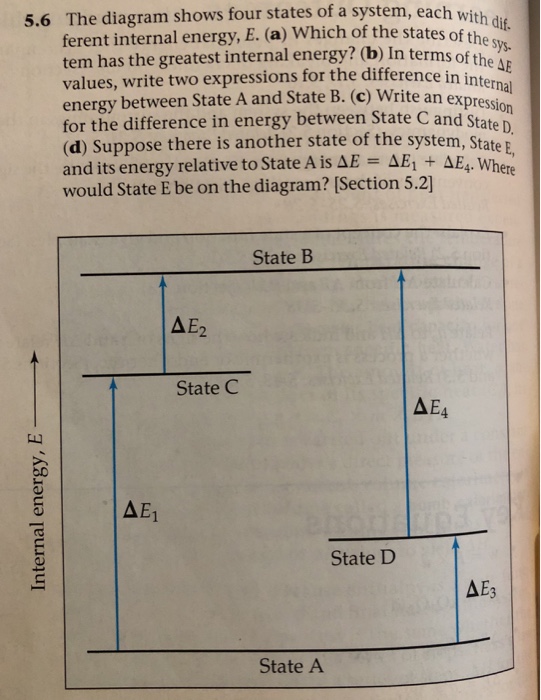
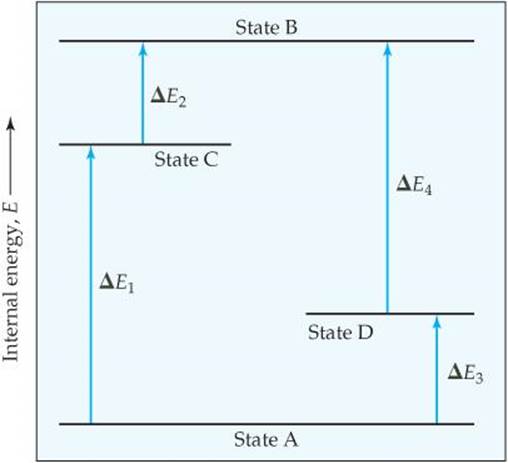
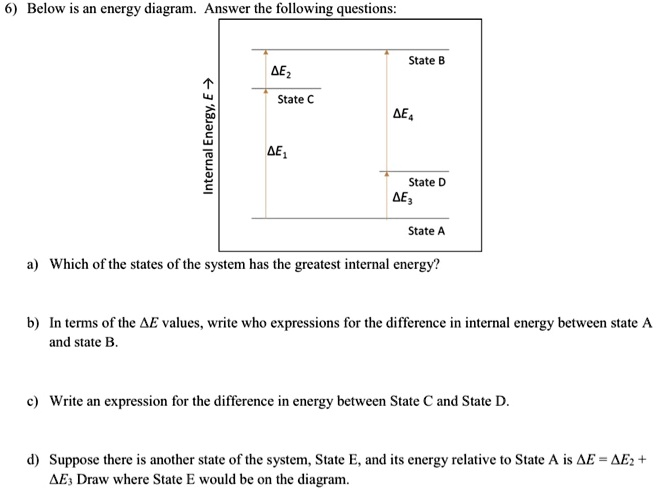
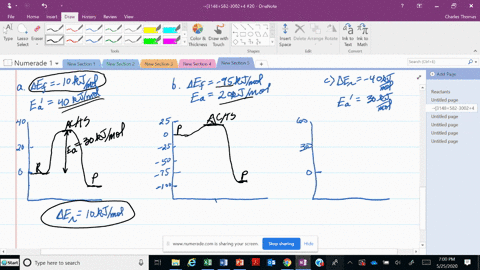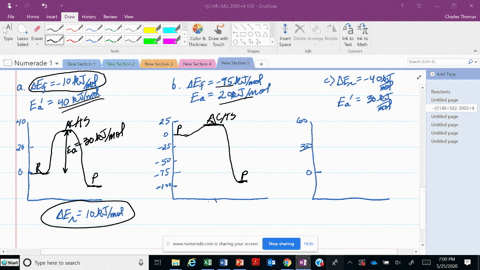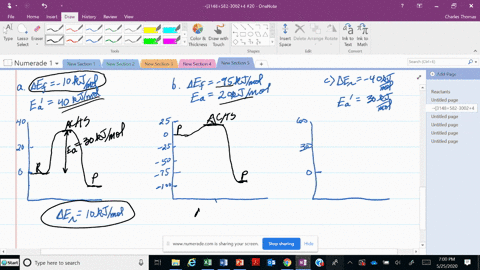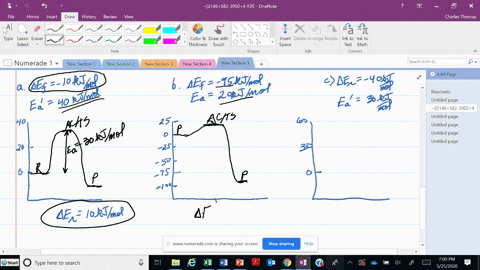
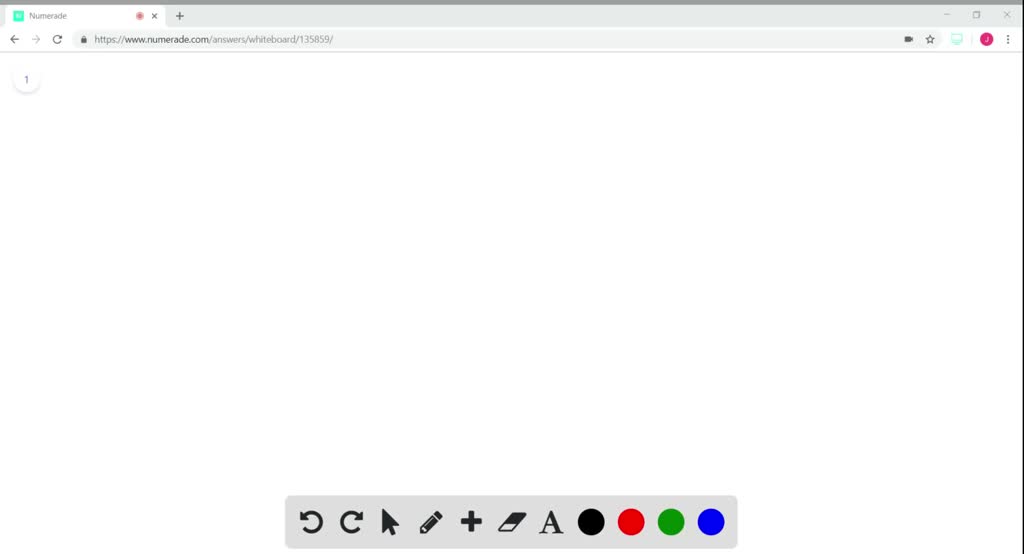
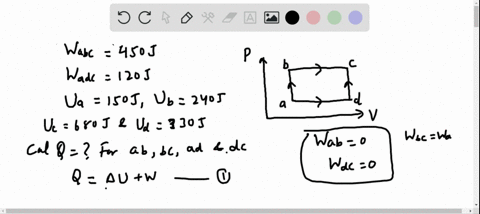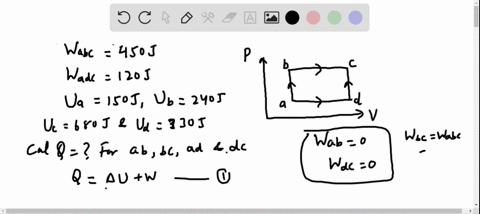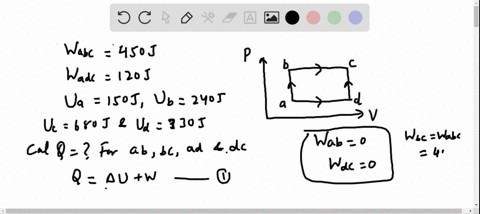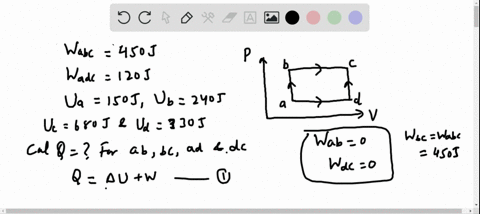
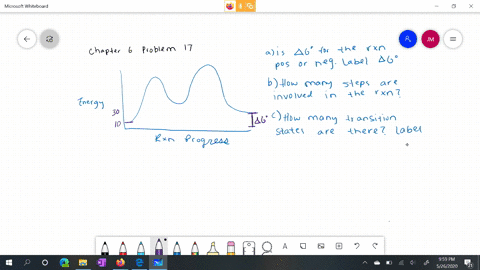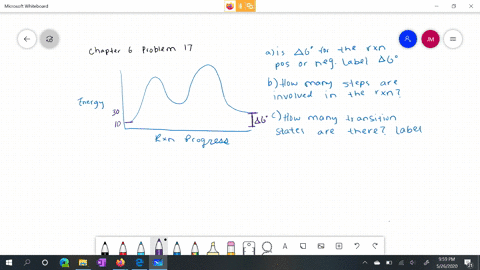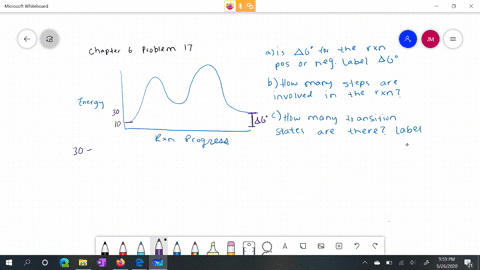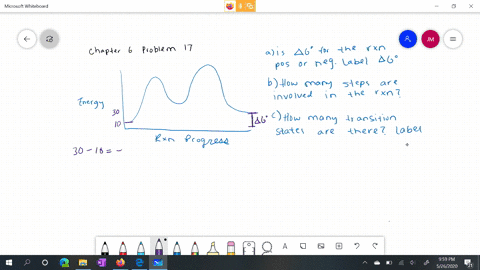
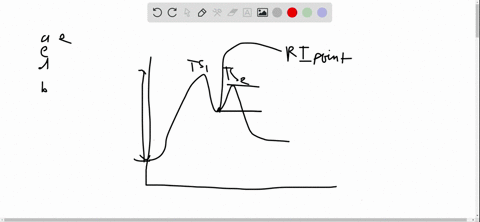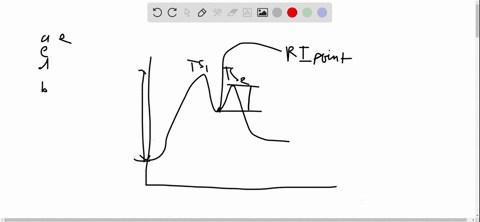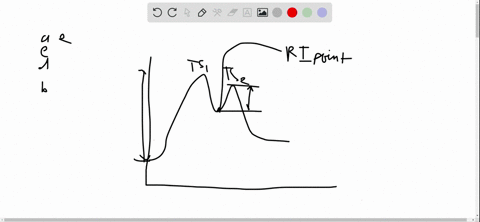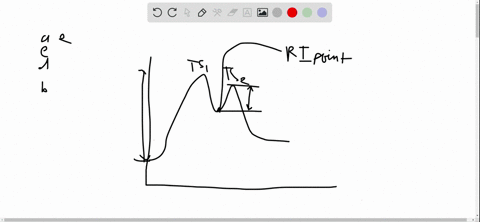
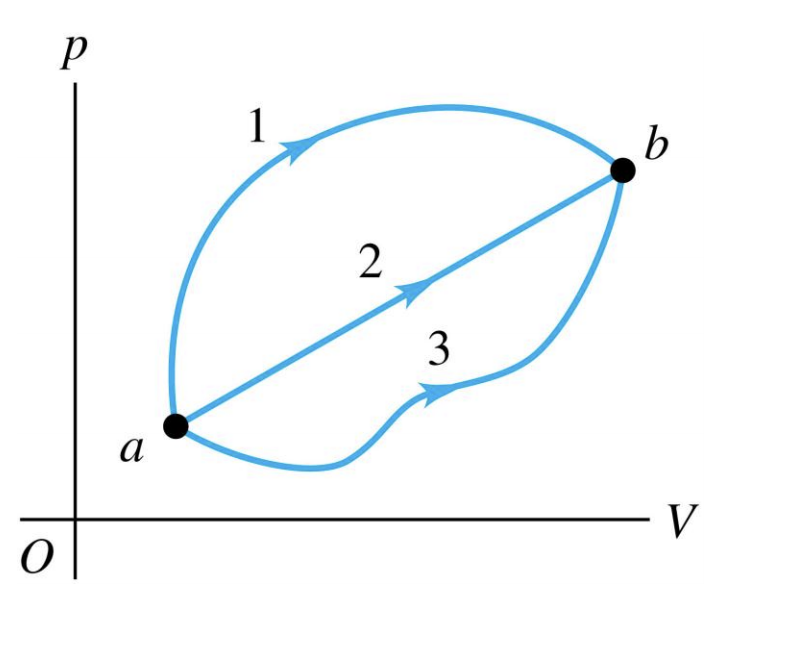
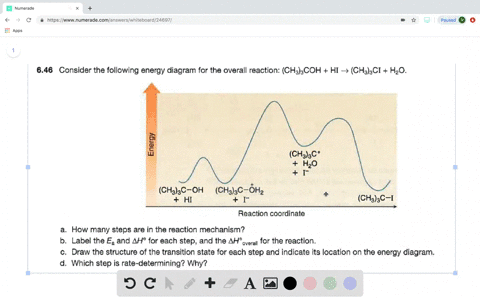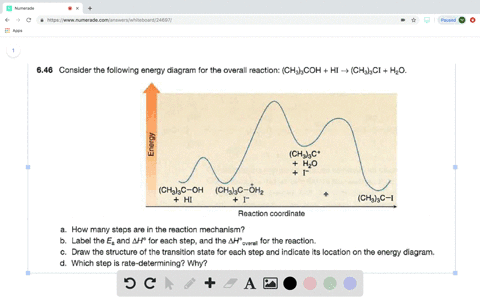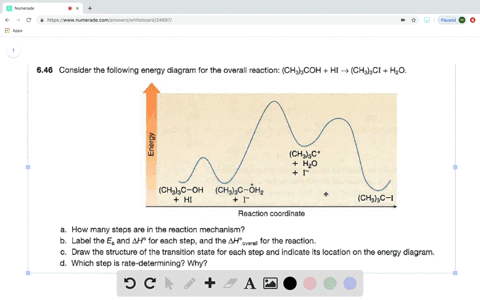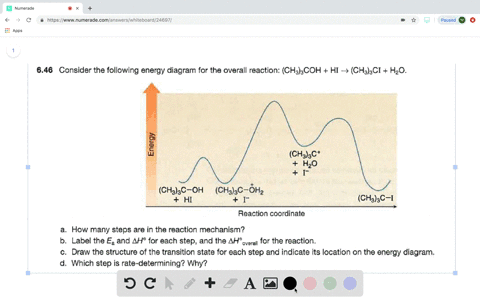
56 The diagram shows four states of a system each with different internal energy E. A system can be taken from state a to state b along any of the three paths shown in the pV diagram. Part A The diagram shows four states of a system each with different internal energy E.
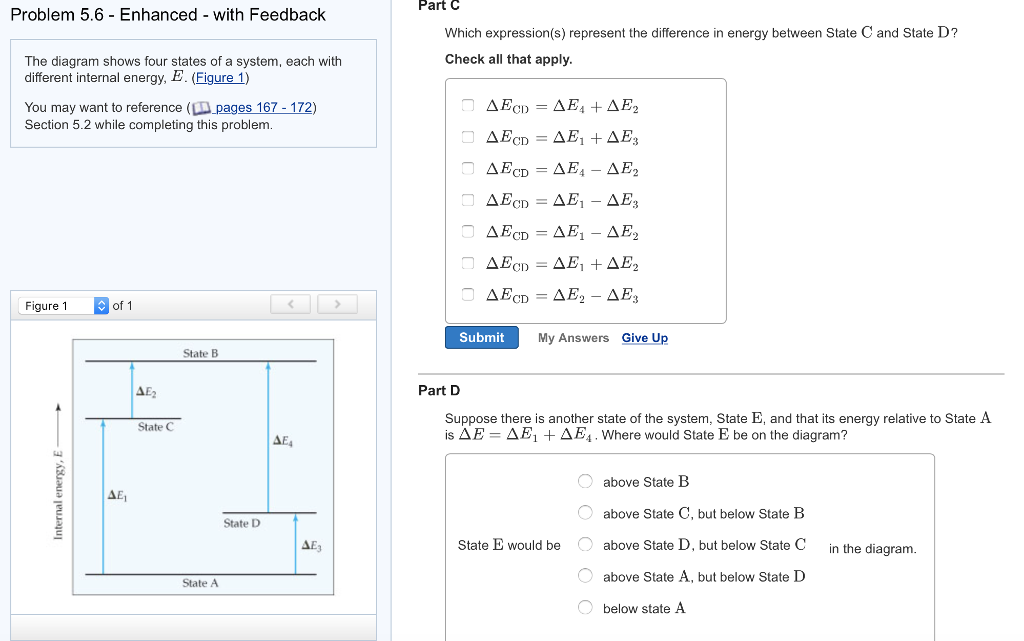
An isolated system cannot exchange heat or work with its surroundings making the change in internal energy equal to zero. AWhich of the states of the system has the greatest internal energy. C Write an expression for the difference in energy between State C and.
A system in an equilibrium state may have some or all of the following kinds of internal equilibria. An uncovered pot of boiling water on a store is an example closed systems systems that can exchange energy but not matter with. Δ𝐸ABΔEAB Write an expression for the difference in internal energy between State A and State B in terms of Δ𝐸3ΔE3 and Δ𝐸4ΔE4.
It does not include the kinetic energy of motion of the system as a whole nor the potential energy of the system as a whole due to external force fields including the energy of displacement of the surroundings of. In thermodynamics a state function is an exact or complete differential that defines the physical condition configuration or state of a system in a manner that is independent in how the system arrived at that state. Internal energy is the sum of potential energy of the system and the systems kinetic energy.
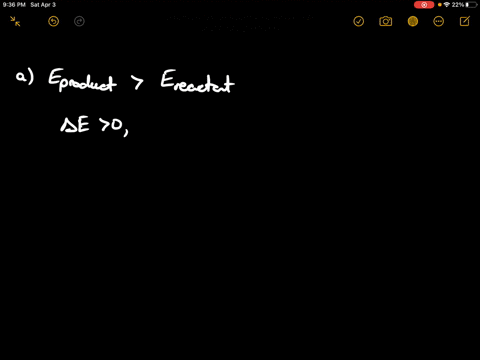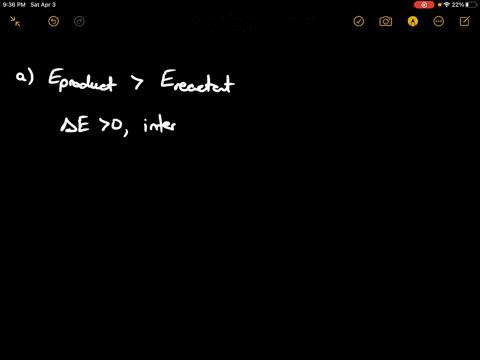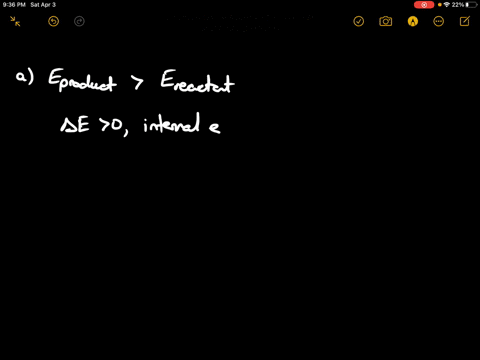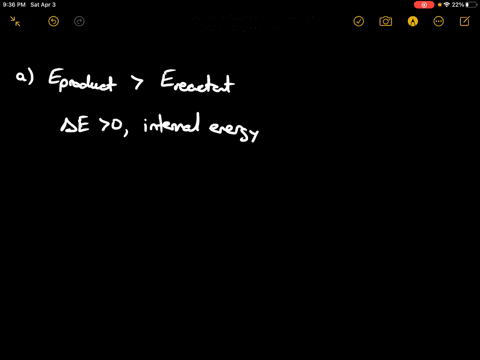
If state b has greater internal energy than state a along which path is the absolute value Q of the heat transfer the greatest. In chemistry and physics internal energy U is defined as the total energy of a closed system.
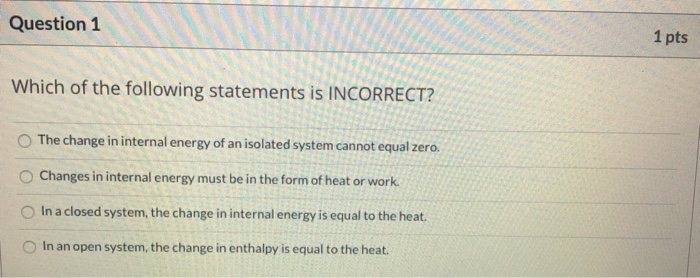
C The total entropy of the universe increases in any spontaneous process.
AWhich of the states of the system has the greatest internal energy. If state b has greater internal energy than state a along which path is the absolute value Q of the heat transfer the greatest. If a property eg enthalpy H is defined as a combination of other state variables then it too is a state variable. The signs of internal energy. The internal energy of a thermodynamic system is the energy contained within it. Because the final temperature is the same the change in temperature is the same as well. Not enough information given to decide. It does not include the kinetic energy of motion of the system as a whole nor the potential energy of the system as a whole due to external force fields including the energy of displacement of the surroundings of. Work is thus done on the system w0.
The signs of internal energy. Because the final temperature is the same the change in temperature is the same as well. C Write an expression for the difference in energy between State C and State D. Internal energy is the sum of potential energy of the system and the systems kinetic energy. The internal energy of a thermodynamic system is the energy contained within it. ΔU isolated system 0. A system such as your food contains chemical energ.

.png)

















Post a Comment for "Which Of The States Of The System Has The Greatest Internal Energy?"